GDPR in Healthcare: A Practical Guide to Global Compliance


Healthcare organizations worldwide must balance patient care with strict data privacy laws. The EU’s General Data Protection Regulation (GDPR) has global implications for GDPR healthcare compliance, extending beyond the EU to any entity handling EU citizens’ personal data. In the healthcare context, GDPR treats health data as a special category, imposing extra safeguards and transparency measures. This practical guide explains when GDPR applies to healthcare, how it differs from HIPAA, and key compliance steps for protecting patient information.
The GDPR’s scope is broad. It governs any “controller or processor” operating in the EU (Article 3(1)) and even non-EU entities offering goods/services to EU individuals or monitoring their behavior (Article 3(2)). This means a US-based telemedicine provider or health app targeting EU users must follow GDPR rules. In short, GDPR applies to all healthcare organizations processing EU individuals’ data, regardless of location, and any entity within the EU or EEA.
GDPR’s material scope covers “personal data related to the physical or mental health of a natural person”. This includes information from medical records, treatment histories, lab test results, genetic profiles, biometric data, and any data revealing someone’s health status. Recital 35 further explains that health data encompasses “any information on disease, disability, risk of disease, medical history, clinical treatment or medical diagnosis”. It covers everything from electronic health records to fitness tracker data if it reveals health information.
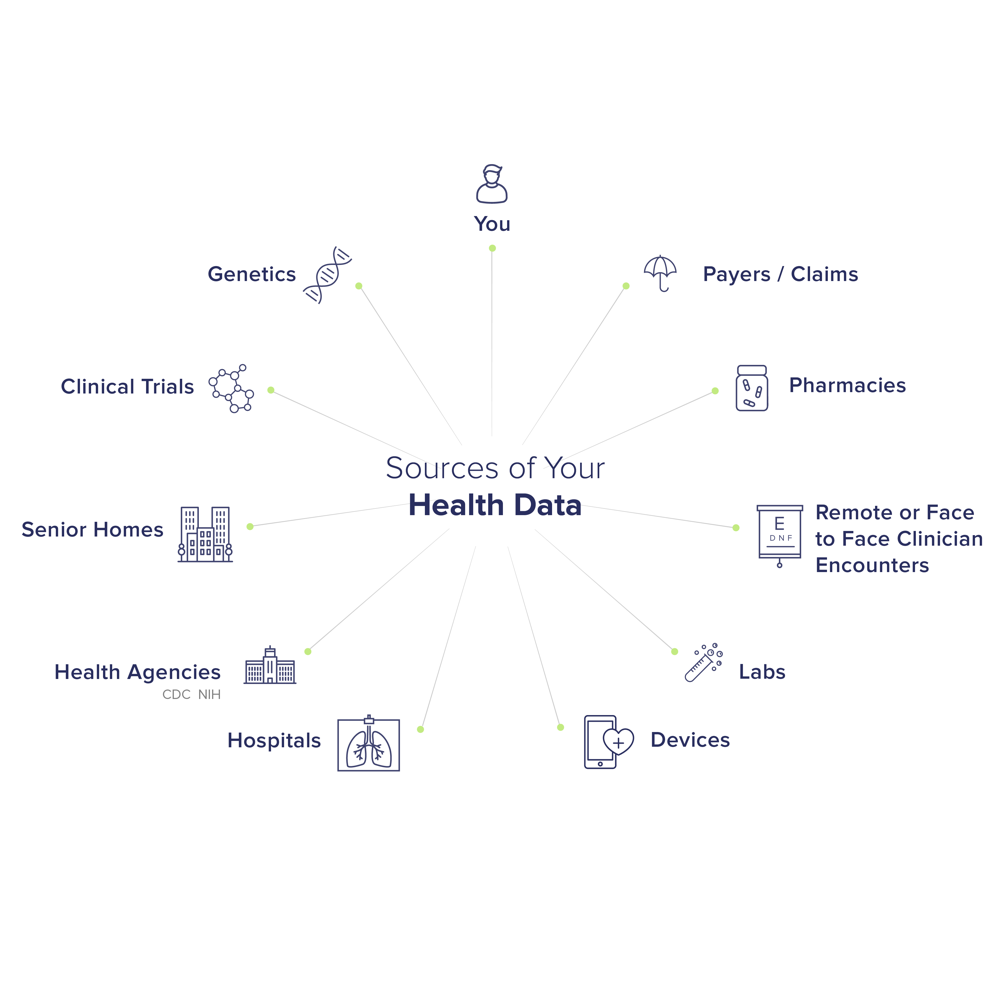
GDPR compliance extends to all entities handling the health data of EU persons. This includes hospitals, medical practices, health insurers, pharmacies, telehealth providers, public health authorities, research institutions, and even third-party vendors (e.g., cloud IT providers, patient portal software companies). Even if a healthcare organization is covered by HIPAA in the US, it must also comply with GDPR when processing EU patient data. Conversely, entities not normally covered by HIPAA (like life insurers, employers, or mobile health apps) will still face GDPR obligations if they collect EU health data.
Many healthcare entities (especially global ones) must navigate both GDPR and HIPAA. Both of them ensure data protection in healthcare. HIPAA is a US federal law focused on safeguarding Protected Health Information (PHI), whereas GDPR is an EU/EEA regulation protecting all personal data (with additional protections for special categories like health data). Here are some of the key differences in GDPR vs HIPAA.
Under GDPR, controllers (e.g., hospitals) determine how and why data is used, while processors (e.g., a cloud provider handling EHRs) follow the controller’s instructions. Both must put appropriate safeguards in place. The GDPR explicitly requires that processing by a processor be governed by a contract specifying each party’s obligations (Article 28). Similarly, HIPAA requires covered entities to sign Business Associate Agreements with any vendor receiving PHI, ensuring the same safeguards apply.
GDPR also introduces the Data Protection Officer (DPO) role in healthcare (required for large-scale/sensitive processing). The DPO must operate independently (no instructions about their duties, protected from dismissal) and report to top management. Under HIPAA, while not called a DPO, covered entities must designate a Privacy Officer (for the Privacy Rule) and a Security Officer (for the Security Rule) to oversee compliance. Healthcare DPOs often work alongside IT security and clinical risk teams, reviewing contracts and SLAs with vendors, and ensuring DPIA coverage and transfer-impact assessments are performed.
GDPR: To process health data (Art. 9), explicit consent is one allowed basis, but not the only one. Article 9(2) permits processing without consent if necessary for healthcare provision or public health (e.g., “processing necessary for medical diagnosis, healthcare treatment or management of health” under law or contract). Still, organizations must document the legal basis (Art. 6 for general processing, plus an Art. 9 condition for special data). Consent under GDPR must be “freely given, specific, informed and unambiguous” and in healthcare contexts, obtaining it can be challenging (especially from incapacitated patients).
HIPAA: There is no GDPR-style “lawful basis” framework. Instead, HIPAA’s Privacy Rule defines permitted uses/disclosures of PHI. For example, healthcare providers can share PHI with other providers for treatment without needing patient authorization. Patient “consent” in HIPAA (sometimes called authorization) is only required for uses beyond standard care (e.g., marketing communications, certain research uses). Thus, HIPAA generally allows more flexibility in routine care: consent isn’t needed every time a patient’s data flows for treatment and billing.
GDPR grants patients (data subjects) a suite of rights: access, rectification, erasure (“right to be forgotten”), restriction, data portability, and objection. Organizations must have processes to honor these rights, ensuring data protection in healthcare. By contrast, HIPAA grants patients a right to access and amend their records and to receive an accounting of disclosures, but it has no erasure or portability requirement. GDPR generally requires transparent communication. Notices must explain all data uses and the patient’s rights. HIPAA requires a simplified Notice of Privacy Practices, but it need not explain rights like erasure or portability.
Both GDPR and HIPAA carry significant enforcement risk. Under GDPR, serious breaches or noncompliance can trigger fines up to €20 million or 4% of annual revenue, plus corrective orders by data protection authorities. Under HIPAA, civil penalties are tiered by negligence level (from ~$100 to $50k per violation) and cap out around $1.8–2.1 million per year for the most egregious violations. HIPAA violations can also lead to criminal charges with jail time (up to 10 years for willful, malicious acts). High-profile healthcare data breaches have led to multi-million-dollar settlements in both regimes, underscoring that patient privacy failures carry steep costs.
When processing patient or health data, healthcare entities must embed GDPR principles into operations:
GDPR Article 9 prohibits processing “special categories” including health data, unless one of the listed conditions applies. Key lawful conditions for healthcare include:
In practice, healthcare providers often rely on the medical necessity exemption for treatment and care, but they must document this basis. Any other use of patient data (e.g., marketing, unrelated research) will typically need explicit patient consent.
GDPR’s foundational principles (Article 5) impose that organizations collect only the minimum health data needed for a specific purpose, and only use it for that purpose. For example, a pharmacy needs minimal patient identifiers and prescription history, but no more. Any collection of unnecessary details (e.g., extensive family history when not needed) violates data minimization.
Purpose limitation means health data gathered for patient care cannot be repurposed (e.g., sold or used for marketing) without a further lawful basis. Data must not be kept longer than needed: GDPR requires defining clear retention schedules and routinely deleting or anonymizing records when no longer necessary. For instance, hospitals might keep medical records for a set number of years per national law and then securely dispose of them.
Article 32 mandates appropriate technical and organizational measures to protect data. In healthcare, this means encrypting electronic health records, pseudonymizing patient data for research, securing network access, and ensuring backups/disaster recovery. Article 32 specifically mentions pseudonymisation and encryption as examples. Thus, a HIPAA-compliant approach (e.g., access controls, audit logs, and encryption) aligns well with GDPR’s security focus. Regular security audits, vulnerability assessments, and incident response drills are also expected under GDPR’s accountability requirements.
Healthcare entities often rely on third-party vendors (EHR platforms, analytics services, telehealth providers). GDPR requires them to conduct due diligence on vendors’ data protection practices and to sign GDPR-compliant contracts (Data Processing Agreements) that mandate security and compliance. Just as HIPAA requires Business Associate Agreements, GDPR requires clear processor agreements. Organizations should monitor vendor compliance (e.g., by requiring audit rights or certifications) and ensure the same obligations cover subprocessors. In short, any entity handling patient data on the organization’s behalf must be held to GDPR standards.
Clinical trials and global research introduce special privacy considerations under GDPR:
Clinical trials frequently involve health data processing at scale. GDPR for healthcare requires clinical research to respect patient rights (informed consent, withdrawal, data erasure in some cases) and often mandates Data Protection Impact Assessments (DPIAs) for high-risk studies. Consent forms should clearly explain data use, retention, and international data sharing. Ethical review boards now often require privacy safeguards (e.g., anonymization for analysis). If a trial spans EU and non-EU sites, researchers must ensure GDPR rules cover all phases (e.g., consents in line with EU standards, appropriate data transfer mechanisms between sites).
Transferring personal health data outside the EU requires GDPR safeguards. Mechanisms include:
To turn theory into practice, healthcare organizations should take concrete steps:
Start by creating a data map. Document what patient data is collected, from where, how it flows (e.g., from clinics to labs to insurance), and who receives it. Article 30 requires maintaining a Record of Processing Activities (RoPA) that lists categories of health data, purposes, recipients, retention periods, and security measures. For each department (e.g., Radiology, Billing, Research), record the types of data processed and legal bases. This inventory is critical for audits and DPIAs.
Under Article 35, a Data Protection Impact Assessment (DPIA) is mandatory when processing health data on a large scale or with new technologies. Any high-risk processing (e.g., genetic profiling, patient tracking, or linking hospital records across countries) should trigger a DPIA. A DPIA should systematically describe the processing activity, assess its necessity and proportionality, identify privacy risks, and outline safeguards. For example, a hospital implementing a new AI diagnostic tool on patient data would perform a DPIA to ensure the risks (like re-identification) are mitigated. Regulators expect DPIAs for major projects involving patient data.
Human error is a major risk. Regular training on GDPR basics (data subject rights, secure handling of PHI, phishing awareness) is essential for all healthcare staff – from doctors to admin to IT. Training should cover the differences between GDPR and HIPAA obligations, the importance of not over-collecting data, and procedures for patient data requests. Customized scenarios (e.g., “what to do if a patient withdraws consent” or “how to securely email test results”) reinforce learning. Leadership must foster a privacy-conscious culture.
Healthcare organizations must have an incident response plan to address data breaches. Under GDPR, any personal data breach must be reported to the supervisory authority within 72 hours of discovery. If patient data is compromised, affected individuals must also be notified without undue delay. HIPAA’s breach rule is longer (60 days for large breaches), but under GDPR, the clock is much shorter. Plans should include detection, containment, risk assessment (does it involve health data?), internal escalation, notification templates, and post-incident review. Testing this plan (e.g., simulations) is a best practice. In short, have a documented data breach response plan so your team can act swiftly to protect patients and comply with regulators.
GDPR recognizes children as having special protection. For online healthcare services or apps aimed at minors, the general rule is that consent of a parent or guardian is required for children under 16 (EU default; Member States can lower to 13). UK GDPR sets the threshold at 13. In pediatric settings, even beyond online services, healthcare providers should verify appropriate consent procedures for adolescents. For example, a mental health app targeting 15-year-olds in Germany would need parental permission under GDPR.
Separately from GDPR, the ePrivacy Directive regulates the confidentiality of electronic communications. Telehealth platforms must secure patient communications (no listening or recording without consent) and manage cookies/tracking. Any app or website that uses cookies to track users’ health-related browsing requires consent under ePrivacy. Medical device connectivity also triggers ePrivacy rules. Healthcare organizations should treat ePrivacy compliance (cookie banners, opt-ins for marketing) as part of overall data protection.
A DPO has a multifaceted role for data protection in healthcare:
Core Tasks: According to GDPR Art 39, the DPO’s tasks include informing and advising on compliance obligations, training and auditing staff, advising on DPIAs, and cooperating with Data Protection Authorities. In healthcare, this translates to things like running workshops on patient data rights, reviewing consent forms, and ensuring research protocols include data safeguards.
Many healthcare organizations already follow security standards like ISO 27001 or the NIST Cybersecurity Framework. GDPR can be integrated with these:
On the international front, organizations also need to consider other data protection laws. For example, Canada’s PIPEDA has some parallels with GDPR. Reviewing differences (e.g., consent requirements under PIPEDA vs GDPR) ensures global strategies work everywhere.
For a cohesive approach, aligning GDPR requirements with existing information security measures and privacy practices makes compliance more efficient and less redundant.
Given the complexity of GDPR in healthcare, many organizations seek outside expertise. DPO Consulting is an expert partner helping organizations with GDPR compliance. GDPR healthcare compliance can feel overwhelming, particularly when it comes to ensuring your privacy team stays current with the latest legal requirements, internal procedures, and technical protocols. That’s where DPO Consulting shines. We are your trusted partner in building in-house expertise through practical, scalable training and coaching tailored to your healthcare needs. We begin with a comprehensive Compliance Audit Services engagement to identify gaps in your privacy program (records, policies, technical controls) and recommend fixes tailored to healthcare.
Our DPO Consulting team specializes in healthcare data privacy. We can help you interpret GDPR articles in clinical contexts, integrate privacy with clinical risk management, and ensure you have processes (consent management, breach response, cross-border protocols) that withstand regulatory scrutiny. With expert support, you can focus on patient care while maintaining robust privacy compliance.
Yes – if a U.S. healthcare provider processes personal data of EU residents or markets services to them, GDPR applies. For instance, a U.S. hospital offering online appointments to EU patients must comply. Even if the hospital is not physically in the EU, GDPR’s Article 3(2) captures any controller targeting or monitoring EU individuals.
Health data means any personal data about physical or mental health status. This includes medical records, diagnoses, test results, genetic information, or any information revealing health conditions. In practice, anything you’d find in a patient’s file (or that reveals illness or treatments) counts as health data.
Yes. For example, a multinational hospital chain may be subject to GDPR when handling EU patient data and HIPAA for US patient data. Even a single patient’s data could be subject to both if they cross borders. When both apply, you must satisfy both sets of rules (often HIPAA’s baseline security plus GDPR’s extra requirements for consent and rights).
GDPR fines can reach the higher of €20 million or 4% of global annual turnover. HIPAA violations carry tiered civil penalties (up to ~$50k per incident) capped at around $1.8–2.1M per year, plus possible criminal charges (jail time for willful violations). In both regimes, settlements and reputation damage can be even more costly.
You can start by describing the processing (what data, why, who, how long). Then assess necessity and proportionality (is this processing needed for patient care or research?). Identify risks to patients (e.g., re-identification, unauthorized access) and list measures to mitigate them (encryption, access controls, consent protocols). Document this assessment thoroughly.
Not necessarily. Under GDPR, explicit consent is one lawful basis, but not the only one. If processing is necessary for treatment or mandated by law, organizations can rely on those legal bases instead of fresh consent. HIPAA similarly allows PHI use for treatment/payment without authorization. However, any secondary use (e.g., research not covered by care) generally requires explicit patient consent under GDPR (or HIPAA authorization in the U.S.).
You can use GDPR-approved mechanisms. Transfers to a country with an EU adequacy decision (or to a US DPF-certified organization) can flow freely. Otherwise, implement Standard Contractual Clauses (SCCs) or Binding Corporate Rules with a Transfer Impact Assessment and extra safeguards (e.g., encryption at rest) as needed. For example, if sending research data from an EU hospital to a U.S. lab, ensure the lab is DPF-certified or sign SCCs and document the transfer rationale.
Investing in GDPR compliance efforts can weigh heavily on large corporations as well as smaller to medium-sized enterprises (SMEs). Turning to an external resource or support can relieve the burden of an internal audit on businesses across the board and alleviate the strain on company finances, technological capabilities, and expertise.
External auditors and expert partners like DPO Consulting are well-positioned to help organizations effectively tackle the complex nature of GDPR audits. These trained professionals act as an extension of your team, helping to streamline audit processes, identify areas of improvement, implement necessary changes, and secure compliance with GDPR.
Entrusting the right partner provides the advantage of impartiality and adherence to industry standards and unlocks a wealth of resources such as industry-specific insights, resulting in unbiased assessments and compliance success. Working with DPO Consulting translates to valuable time saved and takes away the burden from in-house staff, while considerably reducing company costs.
GDPR and Compliance
Outsourced DPO & Representation
Training & Support




To give you 100% control over the design, together with Webflow project, you also get the Figma file. After the purchase, simply send us an email to and we will e happy to forward you the Figma file.
Yes, we know... it's easy to say it, but that's the fact. We did put a lot of thought into the template. Trend Trail was designed by an award-winning designer. Layouts you will find in our template are custom made to fit the industry after carefully made research.
We used our best practices to make sure your new website loads fast. All of the images are compressed to have as little size as possible. Whenever possible we used vector formats - the format made for the web.
Grained is optimized to offer a frictionless experience on every screen. No matter how you combine our sections, they will look good on desktop, tablet, and phone.
Both complex and simple animations are an inseparable element of modern website. We created our animations in a way that can be easily reused, even by Webflow beginners.
Our template is modular, meaning you can combine different sections as well as single elements, like buttons, images, etc. with each other without losing on consistency of the design. Long story short, different elements will always look good together.
On top of being modular, Grained was created using the best Webflow techniques, like: global Color Swatches, reusable classes, symbols and more.
Grained includes a blog, carrers and projects collections that are made on the powerful Webflow CMS. This will let you add new content extremely easily.
Grained Template comes with eCommerce set up, so you can start selling your services straight away.
To give you 100% control over the design, together with Webflow project, you also get the Figma file.